EU commission has approved delays in medical device regulations to avert shortages of lifesaving equipment - the deadline extensions will be even longer than anticipated.
Last month there were a host of concerns surrounding the future availability of medical devices on the EU market with “many manufacturers not sufficiently prepared to meet the robust requirements of the Medical Devices Regulations” due to be introduced in May 2024.
This sparked two main concerns for manufacturers:
The EU commission has since approved the delay in medical device regulation to avoid such shortages. The proposed transition period deadline is extended from 2024 to 2027 or 2028, varying depending on the risk class of the device.
NEW MDR DEADLINES - PER RISK CLASS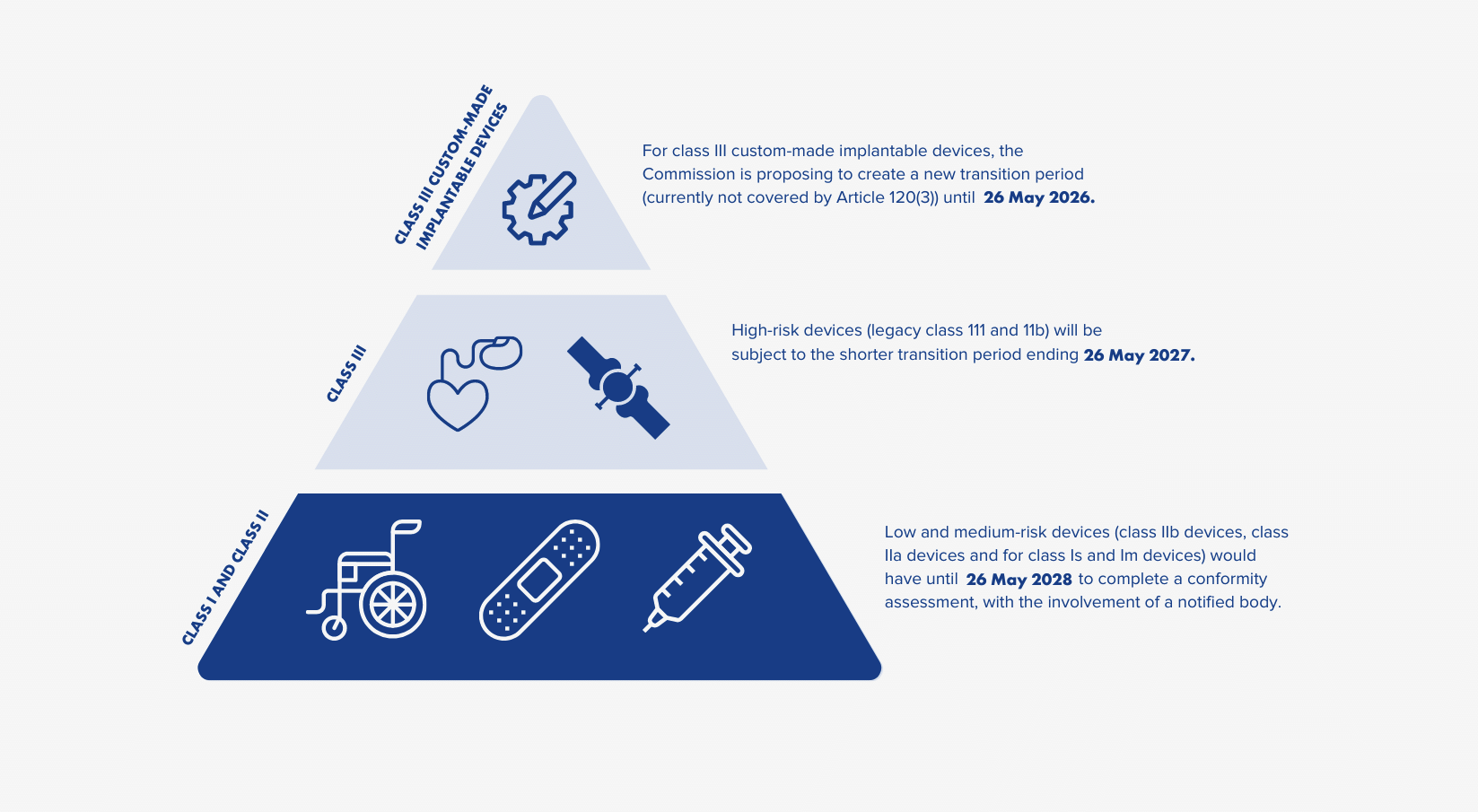
Deletion of sell-off date provision
It is further proposed that sell-off cut-off date will be moved to the 26 May 2025 under both the MDR and (IVDR).
The proposed extension is subject to a number of conditions:
-
The device must not present an “unacceptable risk to health and safety.” (set out in Article 94 and 95 of the MDR)
-
The device must continue to comply with MDD or AIMDD.
-
The device must not have any “significant changes” made in design and intended purpose.
-
By May 26, 2024, the manufacturer must have put in place a Quality Management System (QMS) in compliance with Article 10(9) of the MDR.
-
For the legacy device, the manufacturer must lodge a formal application for an MDR conformity assessment by May 26, 2024, and the parties must sign a written agreement for this assessment by September 26, 2024.
Have you got the teams in place to effectively meet the new MDR deadlines?
To learn more about how Thor Life Sciences can support your business in these exciting but challenging times of hiring, reach out to Aaron Breslin.
Alternatively, to find out more about our services click here: https://www.thor-companies.com/recruitment



.png)

